Source: medgadget.com
Middle ear infections are one of the most common reasons for visits to the pediatrician. However, because the middle ear is hidden from view by the ear drum, physicians must diagnose based on symptoms and a limited physical exam — and when patients have ambiguous or borderline symptoms, accurate diagnosis can be challenging.
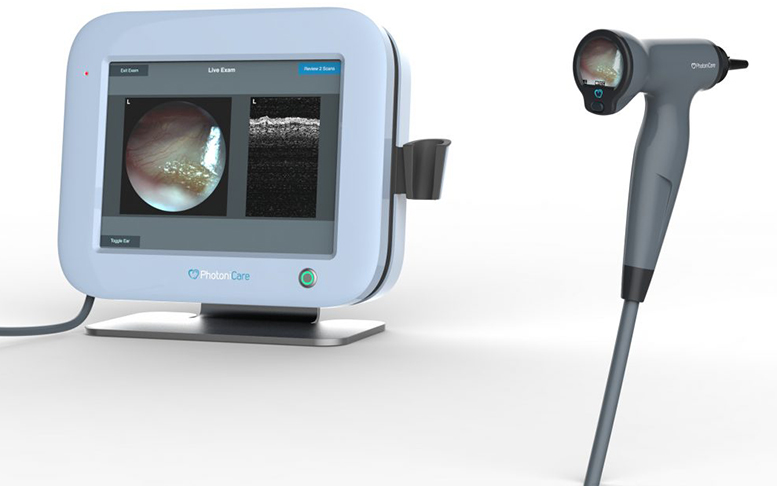
PhotoniCare’s TOMi Scope uses near-infrared light waves to provide 3D views beyond the ear drum into the middle ear, allowing physicians to visualize and better diagnose middle ear infections.
Co-founder and CEO Ryan Shelton, Ph.D. answered a few of our questions about his company’s beginnings, its technology, and what he expects for his firm’s future.
Cici Zhou, Medgadget: Tell me about the founding team members. How did PhotoniCare start, and what was the inspiration?
Ryan Shelton: I founded the company with Stephen Boppart [Professor and Head of the Biophotonics Imaging Laboratory at the University of Illinois at Urbana-Champaign], and Ryan Nolan, an engineer and clinical trial specialist. I was doing postdoctoral research in Dr. Boppart’s medical imaging lab leading a project on an imaging technology with Ryan N. We were exploring a few potential clinical indications and during this same time my oldest son, Jack, now 7 years old, was going through his first year of life. He had 10 office visits with multiple rounds of antibiotics before finally getting a referral to an ENT for surgery. I got to experience first-hand the challenges and frustrations that accompany the current standard of care for ear infections. That experience certainly drove my passion for using our technology platform to improve care for this extremely common disease.
Medgadget: In plain terms, how does the TOMi Scope technology work? Are there studies showing that it is better at helping with diagnosis than traditional otoscopes?
Shelton: The TOMi Scope contains two important imaging modes in one familiar handheld package. It provides a high-quality video otoscope with capabilities for digital export and real-time sharing with parents & patients. It also provides a depth imaging mode that uses near-infrared light to image through the eardrum and directly visualize the contents of the middle ear. Current otoscopes misdiagnose the presence of fluid 50% of the time and give little or no indication of the type of fluid, yet proper diagnosis of both the presence and type of fluid are extremely important for ensuring patients receive proper treatment.
We have several publications on the technology showing the ability to detect and characterize fluid in the middle ear. We have a manuscript currently under review that shows 90%+ sensitivity and specificity for detecting fluid through the eardrum in the pediatric population. The biggest shortcoming for other technologies trying to address this problem is that nearly all of them measure the eardrum in some way. We believe that is the wrong approach. The disease lives in the middle ear, so the middle ear is what we need to assess to make an accurate diagnosis. Unlike competing technologies, we directly visualize the middle ear contents. We are not measuring the eardrum as a proxy for middle ear health.
Medgadget: At what stage is the company today?
Shelton : Today, the company has finalized development of the device and we are in the regulatory process. We keep a lean team of around 5 [full time employees]. We’ve raised less than $2M of private capital (along with $4.5M in grant funds) to get where we are now and should be closing a commercial-stage financing soon. The device has been piloted at 4 clinical sites and we have a long waiting list of additional providers that would like to participate in our upcoming beta program.
Medgadget: What are some of the biggest challenges facing the team, whether it’s on the technology or business side?
Shelton: The team is preparing for a growth phase, so making sure we attract the right team members during that phase will be critical. The team makes and breaks any enterprise, and we are always looking for stellar team members that have a passion for improving healthcare at the front lines. One ever-present hurdle in our industry, and especially in primary care, is market access. We have been working hard to ensure that we have the proper channel partners (we closed a distribution deal in Japan earlier this year) and value proposition to effectively access the market, but that will always be a challenge we are devoting mind power and resources toward.
Medgadget: Looking to the next 5 years, what are the biggest goals for PhotoniCare?
Shelton: We are incredibly excited about the next 5 years. Five years from now we expect to be seen as the next standard of care for ear health in primary care, urgent care, and retail settings. We expect to have additional clinical trials completed and published, and we expect to have changed outcomes for millions of children and adults in [the] US, Japan, and EU. Additionally, one of the great things about our technology platform is that it truly is a platform… We have shown the ability to image the eye, skin, teeth, and oral mucosa in addition to the ear, so we are incredibly excited about the future as we grow our platform and make an enormous impact on efficiency and outcomes at the front lines of medicine.