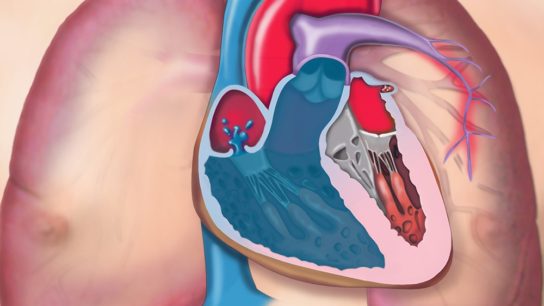
Introduction to Atrial Septostomy
Atrial septostomy is an interventional (often catheter-based) procedure in which a controlled opening or enlargement is made between the right and left atria (the upper chambers of the heart).
The goal is to permit right-to-left shunting of blood-i.e. letting some deoxygenated blood bypass high-resistance pulmonary circulation or decompress a failing right heart-when physiological or anatomical constraints threaten life.
Historically, atrial septostomy has been used especially in neonatal congenital cardiac defects (e.g. transposition of the great arteries, TGA) to allow oxygenated and deoxygenated blood to mix so the body receives some oxygenated blood until definitive surgery.
In more recent decades, a further role has emerged in pulmonary arterial hypertension (PAH) and other severe pulmonary hypertension states: the procedure may act as a palliative "pop-off valve" to relieve right heart pressure, at the cost of some systemic desaturation.
Atrial septostomy is not a cure - it is a bridging, palliative, or symptomatic measure. The decision requires careful balancing of hemodynamics, oxygenation, and risk.
Some quick key points:
-
The common minimally invasive version is balloon atrial septostomy (Rashkind technique)
-
In thicker septa or older neonates, blade/cutting-balloon septostomy or static dilation may be needed.
-
Because the opening may recoil or close over time, repeat procedures or stenting may be required.
-
The procedure carries a risk of hypoxemia, arrhythmias, perforation, and other complications; selecting the proper size and monitoring is critical.
In your blog, you might start with a patient story: e.g. a newborn with TGA and severe cyanosis, for whom septostomy buys time, or an adult with advanced PAH experiencing syncope who receives septostomy to relieve right heart strain.
Causes and Risk Factors (When Atrial Septostomy Becomes Indicated)
Living with the condition after Artificial Disc Replacement (ADR)-whether cervical or lumbar-generally leads to excellent long-term outcomes if recovery guidelines are followed carefully. Patients report restored mobility, reduced pain, and improved quality of life after completing their rehabilitation phase.
Underlying Conditions / Indications
-
Cyanotic Congenital Heart Defects (CHD)
-
Transposition of the Great Arteries (d-TGA) - where the aorta and pulmonary artery are switched, so systemic circulation gets deoxygenated blood. A communication between atria helps mixing.
-
Tricuspid atresia, complex single-ventricle physiology, or other defects where mixing at atrial level is needed for survival.
-
Hypoplastic left heart syndrome (HLHS) with intact atrial septum - fetal or neonatal septostomy may be performed to allow left atrial decompression.
-
-
Pulmonary Arterial Hypertension (PAH) / Severe Pulmonary Hypertension
-
In advanced cases where medical therapy is failing, septostomy may reduce right atrial/right ventricular pressure by shunting blood right → left, thereby decompressing the overworked right heart.
-
It can also serve as a bridge to lung transplantation in selected cases.
-
Some recent data suggests improved survival or hemodynamics in selected patients, though long-term outcomes remain uncertain.
-
-
Other Circumstances / Emerging Uses
-
In adult congenital heart disease with restrictive atrial septum.
-
Rarely in left-heart dysfunction settings or when left-to-right shunt reversal is needed (though this is less common).
-
Risk / Contraindications / Considerations in Candidate Selection
Not everyone is a good candidate. Some risk factors or contraindications include:
-
Very high right atrial pressure (if opening a shunt causes excessive hypoxemia)
-
Severe systemic hypoxemia risk (if the shunt is too large)
-
Left ventricular dysfunction / inadequate left heart output (to handle extra shunt load)
-
Thick or rigid atrial septum (older infants) making balloon septostomy difficult
-
Coagulopathy / bleeding risk
-
Lack of surgical / circulatory support backup
-
Unfavorable anatomy (e.g. proximity to valves, unusual septal structure)
-
Severe systemic disease or multi-organ failure where procedure risk is too high
-
Severe hypoxemia baseline limiting the margin for shunting
Atrial septostomy must be done in a center with high expertise, full imaging and surgical support.
Thus, this section in your blog can describe "Why septostomy is needed" and "What makes a patient eligible or high-risk".
Symptoms and Signs (What Patients Present With / Clinical Manifestations)
Patients with degenerative disc disease (DDD) present with a variety of spinal pain patterns and neurologic symptoms depending on which discs are affected (cervical, thoracic, or lumbar). The condition often manifests as chronic back or neck pain with intermittent exacerbations, sometimes radiating into the limbs due to nerve compression.
In Neonates / Congenital Heart Disease (CHD)
-
Cyanosis (bluish discoloration of lips, skin, nail beds) - severe and often early after birth
-
Hypoxemia / low arterial oxygen saturation
-
Tachypnea / respiratory distress
-
Poor feeding, failure to thrive
-
Lethargy, irritability
-
Acidosis or metabolic derangements
-
Shock or circulatory collapse in severe cases
-
Sometimes differential cyanosis depending on shunt anatomy
-
In TGA, because pulmonary and systemic circulations are parallel instead of series, without mixing via septum or PDA, tissues get poorly oxygenated blood
In Pulmonary Hypertension / Right Heart Failure States
-
Syncope / presyncope - due to acute rises in pulmonary vascular pressure
-
Exertional dyspnea, fatigue
-
Right-sided heart failure signs: jugular venous distension, hepatomegaly, peripheral edema
-
Hypoxemia / desaturation, especially during episodes of increased pulmonary pressure
-
Angina or chest discomfort in some cases
-
In advanced disease, organ hypoperfusion symptoms
In your article, you can provide two patient vignettes:
-
A neonate with TGA who rapidly desaturates unless an atrial communication exists
-
An adult with severe PAH who experiences frequent fainting spells and is deteriorating despite maximal therapy
This helps ground the procedure in real-life clinical need.
Diagnosis of Atrial Septostomy (i.e., diagnosing need / planning)
Diagnosing the need for atrial septostomy and planning the procedure requires a detailed clinical and hemodynamic evaluation, primarily aimed at identifying severe pulmonary arterial hypertension (PAH), specific congenital heart defects, or restricted interatrial communication that compromises oxygenation or cardiac output.
Clinical & Laboratory Evaluation
-
Detailed history: onset, cyanosis, desaturation trends, response to oxygen, symptoms
-
Physical exam: cyanosis, clubbing, signs of right heart strain, murmurs
-
Baseline labs: arterial blood gas (ABG), oxygen saturation, hemoglobin, renal, liver, etc.
-
Cardiac biomarkers, if relevant
Imaging & Hemodynamic Assessment
-
Echocardiography (Transthoracic / Transesophageal Echo, TTE/TEE)
- Evaluate atrial septum anatomy, thickness, presence of patent foramen ovale (PFO) or small ASD
- Assess chamber sizes, pulmonary artery pressures, right ventricular function
- Doppler estimates of pressures and flow -
Cardiac Catheterization / Hemodynamic Study
- Right and left heart catheterization to measure pressures (RA, RV, PA, LA)
- Oxygen step-up measurements, shunt quantification
- Baseline cardiac output / index -
Magnetic Resonance Imaging (MRI) / CT Cardiac Imaging
- In some cases, anatomical delineation -
Electrocardiogram (ECG)
- To detect arrhythmias, conduction abnormalities -
Other supportive testing: e.g. exercise testing, pulse oximetry, mixed venous oxygen levels
Planning & Procedural Considerations
-
Deciding target defect size: balancing shunt vs hypoxemia
-
Use of imaging guidance (bi-plane fluoroscopy, echo) during procedure
-
Choosing appropriate balloon size / blade / dilator devices
-
Ensuring surgical / ECMO / backup support in place
-
Contraindications review and risk assessment
This section could include a workup flowchart: symptoms → echo → hemodynamics → planning → septostomy.
Treatment Options (Atrial Septostomy: Techniques, Alternatives)
Treatment of patients requiring atrial septostomy includes a range of catheter-based and surgical techniques tailored to the patient's anatomy, septal thickness, and underlying condition (e.g., congenital heart disease or pulmonary arterial hypertension). The goal is to create or enlarge a controlled interatrial communication to improve systemic oxygenation and cardiac output.
Techniques / Modalities of Atrial Septostomy
-
Balloon Atrial Septostomy (Rashkind Technique)
- The classic method: through catheterization, a balloon is passed through an existing interatrial communication (PFO or small ASD), inflated, and pulled back into the right atrium to enlarge the opening.
- The easiest, least traumatic, preferred in neonates with thin septum.
- Limitations: older infants, thick septa, or closed foramen ovale may resist balloon tearing. -
Blade / Cutting Balloon Septostomy / Static Dilation
- When balloon septostomy is insufficient, a small blade can create a tear in the septum first, and then balloon expansion is performed.
- Useful in infants older than 4-8 weeks or when septum is thickened.
- Controlled static dilation may follow to maintain the opening. -
Graded Balloon Dilation / Stent-Assisted Septostomy
- Sometimes, the hole is dilated in graded steps, to avoid precipitous oxygen drop or hemodynamic compromise.
- In some centers, stent deployment is used to maintain an interatrial defect permanently, reducing the risk of spontaneous closure. -
Surgical / Open Septostomy
- In rare cases where catheter techniques fail or anatomy is unfavorable, open surgical creation of an atrial septal defect may be done.
Procedural Steps (Typical Balloon Septostomy)
-
Patient preparation and sedation / anesthesia
-
Vascular access (usually femoral venous access)
-
Catheter navigation to right atrium, then across septum to left atrium
-
Balloon inflation and retraction into right atrium (pulling back)
-
Repeated passes / dilations as needed
-
Post-septostomy hemodynamic and oxygenation assessment
-
Monitoring, imaging, closure of access
In more complex approaches, additional cutting or stenting is used.
Alternatives / Adjuncts
-
Medical therapy (in pulmonary hypertension: vasodilators, prostanoids) - septostomy is adjunct/palliative
-
Transplantation (lung or heart-lung) - septostomy can be bridge
-
Other shunt procedures (e.g. Potts shunt) in some centers
-
Closure / modification later - after definitive corrective surgery, septal openings may be closed
You could consider a table comparing techniques: "Technique / Ideal situation / Pros / Cons / Risks".
Prevention, Management & Follow-Up (Around Atrial Septostomy)
Prevention, management, and follow-up care after atrial septostomy are essential for minimizing procedural risks, maintaining hemodynamic stability, and achieving long-term symptom improvement. As the procedure is often performed in critically ill neonates or adults with pulmonary hypertension or congenital defects, meticulous perioperative planning and structured postoperative surveillance are vital.
Prevention (Preventing Septum Closure, Optimizing Outcomes)
-
Use stents or reinforcement in some patients to prevent spontaneous closure of septal opening
-
Graded dilation approach to avoid excessive hypoxia during procedure
-
Careful patient selection and procedure planning
-
Use imaging guidance and controlled inflation to avoid over-sizing the defect
Peri-Procedural / Procedural Management
-
Hemodynamic monitoring
-
Oxygen supplementation and monitoring of saturations
-
Backup circulatory / surgical support (ECMO, surgical standby) if needed
-
Use of echo / fluoroscopy guidance to avoid unintended damage
-
Gradual or stepwise dilation
Post-Septime / Long-Term Management & Surveillance
-
Monitoring saturation / hemodynamics continuously after the procedure
-
Echocardiography follow-up to assess defect size, right/left chamber pressures, shunt flow
-
Re-intervention planning in case of spontaneous closure or restrictive defect
-
Continued therapy for underlying disease (e.g. PAH medications)
-
Assessing performance, symptoms, quality of life
-
Anticoagulation / antiplatelet if needed (depending on stents/use)
Given that septal openings can recoil or partially close, a plan for monitoring and possible repeat procedures is essential.
In your article, you might include a timeline: immediate post-procedure, first 24-72 h, first week, 1 month, long-term.
Complications of Atrial Septostomy
Complications of atrial septostomy can range from minor and transient arrhythmias to serious events such as cardiac perforation, stroke, or death. The overall major complication rate across modern studies varies from 2% to 10%, depending on patient age, anatomy, procedural technique, and operator experience.
General / Procedure-Related Complications
-
Vascular access complications (bleeding, hematoma)
-
Catheter / wire malfunction or entrapment
-
Contrast reaction (if contrast used)
-
Infection (rare)
-
Arrhythmias / conduction disturbances (e.g. AV block, atrial flutter)
-
Embolism / stroke - although many meta-analyses suggest balloon AS is not strongly associated with brain injury risk in some settings
Septostomy-Specific / Mechanical / Hemodynamic Risks
-
Balloon rupture / fragment embolization - balloon may fail during inflation, requiring retrieval
-
Failure of balloon deflation (rare) - requiring intervention to deflate or puncture balloon
-
Cardiac perforation / damage to atrial appendage, septum, pulmonary veins, vena cava, mitral valve injury
-
Excessive shunt / hypoxemia - too large defect can drop systemic oxygen saturation dangerously
-
Right heart decompensation if shunt too large and systemic hypoxia impairs oxygen delivery
-
Spontaneous closure / recoil of the septal defect over time - necessitating repeat procedures
-
Death - risk is small but nonzero, especially early post-procedure in fragile patients
Published data:
-
In older literature series, procedural mortality ranged ~2-3% in some cohorts.
-
In pulmonary hypertension applications, short-term (≤ 48 h) mortality ~4.8%, 30-day ~14.6%, and long-term in some series ~37.7% over several years (mean follow-up ~46.5 months)
-
In a 2024 study of balloon atrial septostomy in TGA, minor complications included transient AV block, atrial flutter, femoral vein thrombosis
When writing this section, include a table: complication, incidence (if known), prevention / management.
Also include "warning signs to monitor after septostomy" (e.g. worsening hypoxia, new arrhythmia, chest pain, hemodynamic instability) so that patients/clinicians know when to act.
Living with Atrial Septostomy (Post-Procedural Life & Long-Term Outlook)
Living with atrial septostomy involves adapting to improved but carefully balanced cardiac physiology. The procedure often brings significant relief in pulmonary arterial hypertension (PAH) or cyanotic congenital heart disease, yet it requires lifelong monitoring, medication management, and lifestyle adjustments to maintain stability and prevent complications.
Immediate Recovery & Monitoring
-
Patients are closely monitored in ICU / step-down setting post-procedure
-
Continuous ECG, saturations, hemodynamic monitoring
-
Serial blood gases and saturation checks
-
Echocardiograms or imaging to assess defect and pressures
-
Adjustments in oxygen therapy, medications as needed
-
Observation for arrhythmias, bleeding, vascular access site
Medium-term & Long-term Life
-
Follow-up imaging / echo / hemodynamics routinely
-
Symptom monitoring: checking for recurrent cyanosis, fatigue, dyspnea, syncope
-
Possibility of re-intervention if the septal opening narrows or closes
-
Ongoing therapy for underlying disease (e.g. PAH): medications (prostanoids, endothelin receptor antagonists, phosphodiesterase inhibitors)
-
Lifestyle adaptation: minimizing hypoxemic stress, regular optimized care
-
Pre-surgical planning: in CHD, the septostomy is often a bridge to definitive anatomical repair (e.g. arterial switch in TGA)
Prognosis & Outcomes
-
In congenital heart disease, septostomy often improves oxygenation, buys time until definitive surgery.
-
In pulmonary hypertension, data show improved hemodynamics, symptom relief, possibly extended survival - but long-term data are limited.
-
Because of the trade-off (some systemic desaturation vs right heart decompression), patient selection and septal sizing are critical
-
Some patients may undergo additional septostomy procedures, or eventual stent closure or repair of the septal defect after correction
Quality-of-Life, Patient Education & Monitoring
-
Educate patients/families about signs of hypoxemia or decompensation
-
Clear plan for follow-up intervals and tests
-
Encourage adherence to therapy (medical, lifestyle)
-
Prepare for potential further interventions
-
Psychological support: living with cyanosis, uncertainties, interventions
You can include a "Frequently Asked Questions" section, e.g.:
-
Will the septal hole stay open forever?
-
Can this cure my pulmonary hypertension?
-
How much will my oxygen saturation drop?
-
What limitations do I have after septostomy?
-
When will the definitive repair happen?
Top 10 Frequently Asked Questions about Atrial Septostomy
1. What is an Atrial Septostomy?
Atrial Septostomy is a cardiac catheterization
procedure in which a small opening (hole) is created between the
right and left atria (upper chambers of the heart).
This allows oxygen-rich and oxygen-poor blood to mix, improving oxygen delivery to the
body and reducing pressure buildup in the right side of the heart.
It is often performed in patients with:
-
Severe Pulmonary Arterial Hypertension (PAH)
-
Congenital Heart Defects like Transposition of the Great Arteries (TGA)
-
Conditions that cause right heart failure due to elevated pulmonary pressure.
The procedure helps improve symptoms and survival in patients who do not respond well to medications.
2. Why is Atrial Septostomy performed?
Atrial Septostomy is performed to improve oxygenation and reduce right heart
strain.
In some heart conditions, especially pulmonary hypertension, the right
side of the heart struggles to pump blood into the lungs due to high pressure.
Creating a controlled hole between the atria allows blood to bypass the
high-pressure pulmonary system, easing the heart's workload.
It can:
-
Improve oxygen delivery to tissues.
-
Reduce shortness of breath and fatigue.
-
Serve as a bridge to lung or heart transplantation in severe cases.
3. Who needs an Atrial Septostomy?
Atrial Septostomy is typically recommended for:
-
Patients with severe pulmonary arterial hypertension (PAH) not responding to medical therapy.
-
Newborns with Transposition of the Great Arteries (TGA) to allow mixing of oxygenated and deoxygenated blood before corrective surgery.
-
Adults with congenital heart defects who need temporary symptom relief.
-
Candidates for heart or lung transplant, where septostomy helps stabilize the patient.
The decision is made after thorough echocardiography, right heart catheterization, and cardiology consultation.
4. How is Atrial Septostomy performed?
The procedure is performed in a cardiac catheterization laboratory under local anesthesia with sedation (or general anesthesia for infants).
Step-by-step procedure:
-
A catheter is inserted through a vein (usually in the groin) and guided into the heart.
-
The catheter reaches the interatrial septum - the wall dividing the left and right atria.
-
A balloon-tipped catheter (Balloon Atrial Septostomy) is inflated to create or enlarge an opening.
-
In some cases, a blade or stent may be used for a more controlled and lasting hole.
-
Blood pressure and oxygen levels are monitored throughout to ensure balance.
The procedure usually takes 30-60 minutes and requires close monitoring in an intensive care setting afterward.
5. What are the types of Atrial Septostomy?
There are two main types of Atrial Septostomy:
-
Balloon Atrial Septostomy (BAS):
-
Most common type.
-
Uses a balloon catheter to enlarge the opening between atria.
-
Commonly performed in newborns with TGA.
-
-
Blade or Stent-Assisted Septostomy:
-
Used in adults or patients with thickened septal walls.
-
A blade or stent is used for a more precise or permanent opening.
-
Often applied in pulmonary hypertension cases.
-
The choice depends on the patient's age, heart structure, and underlying disease.
6. What are the benefits of Atrial Septostomy?
Atrial Septostomy provides significant benefits, including:
-
Improved oxygen delivery throughout the body.
-
Reduced right atrial and ventricular pressure, preventing right heart failure.
-
Better exercise capacity and reduced fatigue.
-
Temporary stabilization before lung or heart transplantation.
-
Enhanced survival in patients with end-stage pulmonary hypertension.
Though it does not cure the underlying condition, it offers critical symptomatic relief and can improve quality of life.
7. What are the possible risks or complications?
While generally safe when performed by experienced cardiologists, Atrial Septostomy carries some risks:
-
Bleeding or blood vessel injury during catheter insertion.
-
Heart rhythm abnormalities (arrhythmias).
-
Excessive shunting leading to low oxygen levels.
-
Stroke or blood clots (rare).
-
Sudden drop in blood pressure.
-
Infection or cardiac tamponade (very rare).
Careful patient selection, real-time imaging guidance, and experienced operators minimize these risks.
8. How long is the recovery period after Atrial Septostomy?
Recovery depends on the patient's condition and reason for the procedure.
-
Hospital stay: Usually 1-3 days for monitoring.
-
Observation: Continuous ECG and oxygen monitoring are done to assess shunt effectiveness.
-
Activity: Patients can resume light activities within a few days, but strenuous exercise is restricted initially.
-
Follow-up: Regular echocardiograms are needed to check the shunt's size and effect on heart pressures.
If performed as a bridge to transplantation, patients remain under close supervision until further treatment.
9. How successful is Atrial Septostomy?
The success rate of Atrial Septostomy is high when performed on well-selected patients.
-
In newborns with TGA, it can be life-saving, improving oxygenation until definitive surgery.
-
In pulmonary hypertension patients, it often relieves symptoms and extends survival.
Long-term outcomes depend on the underlying disease and ongoing treatment with medications such as vasodilators or anticoagulants.
10. Is Atrial Septostomy covered by insurance?
Yes. Most major insurance companies and government health
schemes cover Atrial Septostomy when it is medically indicated - such as
for congenital heart defects or pulmonary hypertension.
Coverage typically includes:
-
Hospital and cardiac lab charges
-
Surgeon and cardiologist fees
-
Catheter and device costs
-
Intensive care monitoring
It's important to confirm with your insurance provider and hospital billing team before scheduling the procedure.


