Introduction to Bidirectional Glenn (BD Glenn)
The Bidirectional Glenn (BD Glenn) procedure is a critical palliative surgery in
the management of children born with complex congenital heart defects
characterised by a “single ventricle” physiology—meaning one of the heart's
lower chambers is either absent or severely under-developed and cannot
effectively pump blood on its own.
In a typical heart, one ventricle (the right) pumps blood to the lungs for
oxygenation, and the other (the left) pumps oxygen-rich blood out to the body.
But in single-ventricle defects, the remaining ventricle must handle both roles
(lungs + body), which places a heavy volume load on it and leads to
inefficiencies and eventual complications. The Glenn procedure helps by
redirecting part of the blood flow (specifically from the upper body via the
superior vena cava) directly into the pulmonary arteries, bypassing the
ventricle and reducing the workload.
Put simply: the BD Glenn is usually the second stage in a
three-stage surgical strategy (Stage I often being the Norwood procedure, Stage
II being the Glenn, and Stage III being the Fontan procedure) for single
ventricle defects.
The goals of the Glenn are to:
-
Reduce the volume load (“work”) on the single ventricle, allowing it to remodel and function more efficiently.
-
Improve oxygenation of the blood (by sending more deoxygenated blood to the lungs) and thereby improve systemic oxygen saturation.
-
Simplify the heart's workload ahead of the final repair (Fontan) and improve long-term outcomes.
Over recent decades improvements in surgical technique, perioperative care and
patient selection have made the BD Glenn procedure a relatively safe and
effective stage in the management of single-ventricle congenital heart
disease.
In the rest of this article we will look at causes/risk, symptoms/signs,
diagnosis, treatment options, prevention/management, complications, and what
life is like with and after the Glenn procedure.
Causes and Risks of Conditions Requiring a BD Glenn
Underlying Causes
It's important to clarify that the BD Glenn is not itself a disease, but a surgical treatment for certain congenital heart defects (CHDs). Thus “causes and risks” in this context refer to the underlying defects and to the risks for needing the procedure.
Some of the congenital heart defects that lead to the need for a Glenn procedure include:
-
Hypoplastic Left Heart Syndrome (HLHS) — where the left side of the heart is under-developed and cannot pump effectively.
-
Tricuspid Atresia — absence or malformation of the tricuspid valve, severely limiting right ventricular output.
-
Double Inlet Left Ventricle or other unbalanced atrioventricular canal defects — where both inflows go to one ventricle or the other ventricle is hypoplastic.
-
Pulmonary atresia with intact ventricular septum or other forms of single-ventricle physiology.
In such situations the circulation is abnormal: the heart often must pump deoxygenated blood plus oxygenated blood mixed together, the volume load is high, and the physiology is one of parallel circuits rather than the efficient series circuit of a normal heart. The Glenn shifts this toward a more efficient pattern.
Risk Factors That Affect Timing & Outcomes
Factors that increase the risk of poorer outcomes (or complications) with the Glenn procedure include:
-
Elevated pulmonary vascular resistance (PVR) or pulmonary hypertension — because the Glenn circulation relies on passive flow of venous blood into the pulmonary arteries.
-
Significant ventricular dysfunction (single ventricle not functioning well) or atrioventricular valve regurgitation.
-
Younger age at operation (especially < 3 months) or delayed timing (> 5 months) has been associated with worse outcomes.
-
Pre-existing complications from Stage I surgery (e.g., prolonged recovery, residual lesions).
Why the Procedure Is Needed
Because the existing ventricle is overloaded and because the options for full repair (Fontan) may not yet be safe or optimal at a young age, the Glenn provides a "bridge" by adopting part of the circulation, thereby both improving oxygenation and reducing volume load.
Symptoms and Signs of Single-Ventricle Physiology (and Indications for a BD Glenn)
Symptoms & Clinical Features
Children with defects requiring a Glenn may display:
-
Cyanosis (bluish discoloration of skin/lips) due to reduced oxygen saturation.
-
Fatigue, poor feeding, growth failure (in infants) because the heart must work very hard.
-
Shortness of breath, rapid breathing or difficulty exhaling if there is congestive overload.
-
Signs of heart failure: sweating with feeding, liver enlargement, fluid accumulation (pleural effusion, ascites) in advanced cases.
Physical Examination Findings
-
A heart murmur or abnormal heart sounds depending on the defect.
-
Signs of volume overload: cardiomegaly (enlarged heart on imaging), possible hepatomegaly.
-
Cyanosis and low oxygen saturations (measured by pulse oximetry).
-
In the period after Glenn surgery: increased central venous pressure reflected by facial or upper-body swelling, pleural effusions or prolonged chest tube output may be seen.
When the BD Glenn Procedure Becomes Indicated
Indications for the Glenn usually occur when the child is stable enough from
Stage I surgery and when growth and pulmonary vascular resistance permit.
Typical timing is around 3-6 months of age, though it may vary.
The decision is based on:
-
Adequate pulmonary artery anatomy and acceptable pulmonary vascular pressures.
-
The child having reached a size and physiological condition where the next stage of palliation is appropriate.
-
Oxygen saturation falling due to shunt restriction or ventricular overload.
Diagnosis and Pre-operative Evaluation for the BD Glenn
Diagnostic Work-up
Before proceeding with a Glenn procedure, a comprehensive evaluation is required including:
-
Echocardiography: to assess anatomy, ventricular function, valve function, shunts, pulmonary artery anatomy.
-
Cardiac catheterisation: to measure pressures in the pulmonary arteries, estimate PVR, evaluate collaterals or AV valve regurgitation.
-
MRI/CT angiography in some centres to assess pulmonary artery size, anatomy, and venous connections.
-
Laboratory tests: baseline organ function (liver, kidneys), coagulation profile, evaluation for other comorbidities.
-
Pre-operative planning: assessing the child's growth, nutrition, current shunt or band status, and readiness for surgery.
Key Criteria for Proceeding with Glenn
-
Acceptable pulmonary artery pressures (mean PA pressure often < 18 mmHg) and PVR within acceptable range (often < 2-3 Woods units) in many institutions.
-
Adequate pulmonary artery anatomy and absence of significant pulmonary artery stenosis or obstruction.
-
Controlled ventricular function and AV valve regurgitation.
-
No uncontrolled systemic led shunts or collateral vessels causing pulmonary over-circulation.
-
Age and size: typically 4-6 months (in many but not all institutions).
Pre-operative Preparation
-
Optimise nutrition, address any infections, improve growth.
-
Manage any residual shunts or collaterals (coil occlusion if needed).
-
Plan for cardiopulmonary bypass or off-pump strategy depending on anatomy and institutional protocol.
-
Family education regarding surgery, risks, anticipated ICU stay, and long-term follow-up.
Treatment Options (Including the BD Glenn)
Staged Surgical Strategy
For single-ventricle physiology, the usual treatment strategy is staged:
-
Stage I: Early neonatal surgery (Norwood or other initial palliation) to establish survival and initial circulation.
-
Stage II: The Bidirectional Glenn procedure (our focus) - redirecting upper-body venous return to the pulmonary arteries and unloading the ventricle.
-
Stage III: The Fontan procedure - routing the inferior vena cava return directly to the pulmonary arteries (completing the circulation) when the child is older (often 18 months to 3 years).
Details of the BD Glenn Procedure
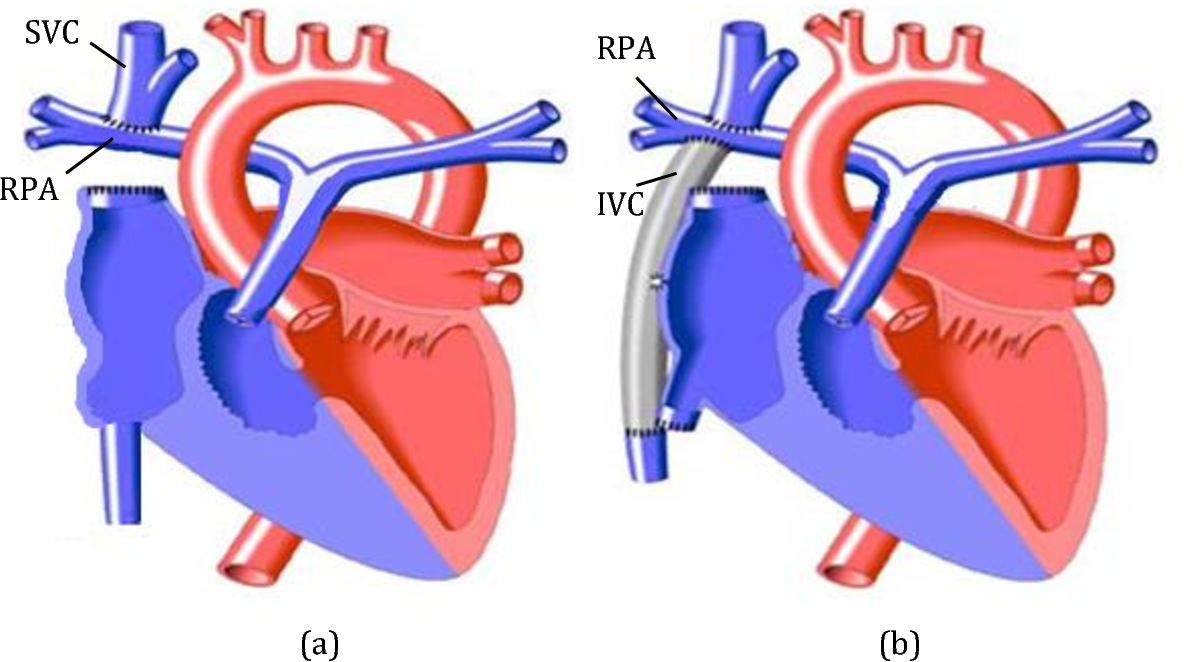
-
The surgeon disconnects the superior vena cava (SVC) from the right atrium and connects it (anastomoses it) to the right (or both) pulmonary arteries so that deoxygenated blood from the upper body flows passively into the lungs.
-
The previously placed systemic-to-pulmonary shunt (from Stage I) may be removed or modified.
-
It may be done with or without cardiopulmonary bypass depending on centre and patient anatomy.
-
Post-operatively, the child will need intensive monitoring, often in an ICU setting, for several days.
Advantages / Goals
-
Reduced volume load on the single ventricle, which helps improve ventricular function over time.
-
Improved systemic oxygen saturation and relief of cyanosis.
-
Better preparation and outcomes for eventual Fontan procedure (if planned).
Alternative or Adjunct Options
-
Some centres may perform a “hemi-Fontan” instead of classic Glenn.
-
Other catheter-based or hybrid approaches may be used in select cases, especially in high-risk infants.
-
In some cases where a Fontan is not feasible, the Glenn may serve as the long-term palliation.
Timing and Decision Making
-
Timing is critical: too early may increase risk; too late may allow complications (ventricular dysfunction, elevated PVR) to develop. Studies show optimal age around 3-6 months for many patients.
-
Decision making involves multidisciplinary team: paediatric cardiologist, cardiothoracic surgeon, intensivist, anaesthesiologist, and others.
Prevention and Management Around the BD Glenn and Long-Term Care
Prevention (in the context of staged palliation)
While one cannot “prevent” congenital heart defects that require a Glenn, certain practices and management steps help optimise outcomes:
-
Early detection of congenital heart defects prenatally (where possible) and timely referral to specialised centres.
-
Careful monitoring of infants with single-ventricle physiology to identify evolving issues (e.g., increasing cyanosis, poor growth, elevated pressures).
-
Control of infections, maintaining good nutritional status, optimising growth - delays in growth or illness can negatively affect timing and outcome.
-
After Stage I surgery, monitoring for pulmonary artery growth, stenosis, collateral formation and ensuring adequate pulmonary vascular development.
Management Before and After the Glenn
-
Pre-operative management: optimisation of heart and lung status, treatment of residual lesions, coil occlusion of collaterals if required.
-
Post-operative care: ICU monitoring, ventilator support as needed, fluid management, prevention of pleural effusions, physiotherapy, nutritional support.
-
Long-term follow-up: Regular visits with congenital cardiology, echocardiography, periodic cardiac catheterisations or MRI as needed. Monitoring for arrhythmias, ventricular function, valve regurgitation, caval and pulmonary artery flow.
-
Lifestyle management: ensuring appropriate growth and development, controlling factors that elevate pulmonary pressures (e.g., lung disease, sleep-apnoea, obesity) and encouraging physical activity within safe limits as advised by the cardiology team.
-
Family support and education: preparing the family for lifelong follow-up, potential need for further surgeries (Fontan or even transplantation) and management of complications.
Complications of the BD Glenn Procedure
While the bidirectional Glenn is an important and often life-saving surgical stage, it is not without risks and potential complications.
Short-Term / Post-Operative Complications
-
Pleural effusions (fluid around the lungs) or ascites (fluid in the abdomen) due to elevated venous pressures.
-
Thrombosis in the SVC or pulmonary arteries, leading to obstruction of the cavopulmonary connection.
-
Elevated superior vena cava pressure causing facial or upper-body swelling, or venous congestion.
-
Arrhythmias or conduction abnormalities.
-
Low oxygen saturation or persistent cyanosis if the pulmonary blood flow is inadequate or there is competition from collaterals.
-
Bleeding, infection, or complications related to cardiopulmonary bypass if used.
Long-Term / Late Complications
-
Progressive ventricular dysfunction or atrioventricular valve regurgitation that may limit further palliation.
-
Development of pulmonary arteriovenous malformations (especially in partial cavopulmonary circulations) leading to desaturation.
-
Thromboembolic events (clots) or stroke risk in some patients.
-
Protein-losing enteropathy or plastic bronchitis in the later Fontan circulation era — though more associated with the Fontan, some risk factors may become evident after Glenn.
-
Need for re-intervention (catheter or surgical) for stenosis of the Glenn anastomosis, pulmonary artery stenosis, or collateral vessel issues.
-
In some cases where the Glenn fails or cannot be completed toward Fontan, transplantation might be required.
Risk Factors for Poor Outcome
Studies have identified factors such as younger age at Glenn (< 3 months), elevated preoperative PVR, AV valve regurgitation, and prior prolonged ICU stay as predictors of worse outcomes.
Mitigating the Risks
-
Careful patient selection and timing.
-
Optimising pulmonary artery anatomy and pressures pre-operatively.
-
Vigilant post-operative monitoring and early intervention for complications.
-
Long-term surveillance to detect problems early.
Living with the Condition of Single-Ventricle Physiology & After the BD Glenn
Quality of Life & Long-Term Outlook
-
Many children undergoing the Glenn procedure go on to have good functional status, particularly if the subsequent Fontan (or equivalent) is successful. The Glenn is a bridge, not necessarily the final step.
-
Life after Glenn still involves lifelong follow-up with a congenital cardiologist, regular imaging/studies, and often restrictions or guidance on exercise, activity, and sometimes lifestyle.
-
Some exertional limitation, abnormal oxygen saturation, or fatigue may persist, especially if the circulation remains “unbalanced”.
-
Growth and neurodevelopmental outcomes: Because these children often had early surgeries and complex physiology, monitoring growth, development and supporting with therapies (physio, occupational, speech) is important.
Practical Advice for Families and Patients
-
Educate families about recognising warning signs: increased cyanosis, feeding/feeding intolerance in infants, shortness of breath, arrhythmia symptoms, swelling (pleural/abdomen), or decreased exercise tolerance.
-
Encourage normal childhood activities as per cardiologist advice, while being mindful of limitations. Many children do attend school, play with friends, and lead fulfilling lives.
-
Emphasise good general health: respiratory infections can place extra load on the circulation; so immunisations, prompt treatment of lung/airway infections, avoidance of second-hand smoke are key.
-
Nutrition is vital: adequate caloric intake, growth monitoring, and in older children healthy weight management (obesity may worsen ventricular/vascular strain).
-
Transition to adult congenital heart disease care: As more children with Glenn/Fontan procedures survive into adolescence and adulthood, planning for transition to adult congenital heart disease (ACHD) clinics is important.
-
Psychological and social support: The stress of surgeries, hospitalisations and long-term medical care can affect not only the child but the family — access to support groups and counselling may help.
What to Expect During Follow-Up
-
Regular clinic visits: often every 6-12 months (more frequently initially) with cardiac imaging (echo, sometimes MRI), ECGs, exercise tests in older children.
-
Monitoring for arrhythmias: Holter monitoring or event monitors may be used.
-
Catheterisation: Some patients may require cardiac catheterisation for assessment or intervention (e.g., coil occlusion of collaterals, balloon/stent of stenoses) as they grow.
-
Exercise guidance: Many children are encouraged to have regular physical activity; tailored by cardiologist.
-
Lifelong awareness: Even after a successful Glenn and Fontan, some risk remains for late complications, so lifelong cardiology follow-up is necessary.
Top 10 Frequently Asked Questions about BD Glenn
1. What is the BD Glenn (Bidirectional Glenn) procedure?
The Bidirectional Glenn (BD Glenn) procedure is a surgical operation that connects the superior vena cava (SVC) — the large vein carrying blood from the upper body — directly to the right pulmonary artery.
This allows blood from the upper part of the body to flow passively into the lungs for oxygenation, bypassing the right ventricle of the heart.
The BD Glenn is typically performed as the second stage of surgical correction for children born with single-ventricle congenital heart defects, such as:
-
Tricuspid atresia
-
Hypoplastic left heart syndrome (HLHS)
-
Pulmonary atresia
-
Double inlet left ventricle (DILV)
It is usually performed when the child is 4 to 6 months old, after the Norwood procedure and before the Fontan procedure.
2. Why is the BD Glenn procedure performed?
The BD Glenn procedure is performed to improve oxygenation and reduce strain on the single functioning ventricle in children with complex congenital heart defects.
By redirecting venous blood directly to the lungs:
-
It reduces the heart's workload, since the ventricle no longer pumps blood to both body and lungs.
-
It improves oxygen levels in the blood.
-
It prepares the circulation system for the final stage (Fontan procedure), which completes the separation of oxygenated and deoxygenated blood.
In essence, the BD Glenn is a palliative (staged) surgery that helps the child grow and gain strength before the next corrective operation.
3. At what age is the BD Glenn procedure performed?
The BD Glenn surgery is typically performed when a baby is 4 to 6 months old, depending on:
-
The baby's growth and weight.
-
Oxygen saturation levels in the blood.
-
Heart anatomy and function as evaluated by echocardiography and cardiac catheterization.
Performing it too early may cause complications due to high pulmonary pressure, while delaying it too long may lead to low oxygen levels and heart strain.
4. How is the BD Glenn procedure performed?
The BD Glenn procedure is an open-heart surgery performed under general anesthesia by a pediatric cardiac surgeon.
Surgical steps include:
-
A midline incision is made on the chest to access the heart.
-
The child is placed on a heart-lung bypass machine to maintain blood circulation.
-
The superior vena cava (SVC) is disconnected from the right atrium.
-
The SVC is then attached to the right pulmonary artery, allowing blood to flow directly from the upper body to the lungs.
-
The surgeon checks for proper flow and closes the chest once stable.
The procedure takes around 4-6 hours, followed by intensive postoperative care.
5. What happens after the BD Glenn surgery?
After surgery, the child is moved to the Pediatric Intensive Care Unit (PICU) for close monitoring.
Postoperative care includes:
-
Ventilation support for 1-2 days until breathing stabilizes.
-
Pain management and antibiotics to prevent infection.
-
Monitoring oxygen levels, blood pressure, and fluid balance.
-
Gradual feeding and physical activity after 3-5 days.
The average hospital stay is 1-2 weeks, depending on recovery
progress.
Parents are taught how to monitor the child's oxygen levels and recognize signs of
complications before discharge.
6. What are the potential risks or complications of BD Glenn surgery?
Like all major heart surgeries, BD Glenn carries some risks, though it is considered safe and effective when performed by experienced cardiac surgeons.
Possible complications include:
-
Bleeding or infection after surgery.
-
Arrhythmias (irregular heart rhythms).
-
Pleural effusion (fluid around the lungs).
-
Superior vena cava syndrome (swelling due to vein blockage).
-
Low oxygen levels if pulmonary blood flow is restricted.
Most of these are manageable with prompt medical care. The surgical success rate exceeds 90% in specialized pediatric cardiac centers.
7. How successful is the BD Glenn procedure?
The BD Glenn surgery has a high success rate (over 90%) and offers significant improvement in:
-
Oxygen saturation levels (typically 75-85% post-surgery).
-
Cardiac efficiency and function.
-
Quality of life and growth in infants awaiting the Fontan procedure.
With proper follow-up, most children recover well and continue to thrive until they are ready for the Fontan completion, which is usually performed between 2-5 years of age.
8. What is life like after BD Glenn surgery?
After a successful Glenn procedure:
-
The child usually has good oxygen levels and improved stamina.
-
Regular follow-ups with a pediatric cardiologist are essential to monitor heart function.
-
Medications such as aspirin or anticoagulants may be prescribed to prevent blood clots.
-
Parents are advised to ensure adequate hydration and avoid dehydration, which can increase blood viscosity.
Most children can grow, play, and attend school normally with minimal restrictions before undergoing the Fontan surgery.
9. How should families prepare for the surgery?
-
Pre-operative assessment is critical: the cardiology team will perform imaging (echocardiography, MRI), catheterization to assess anatomy, measure pulmonary artery pressures and vascular resistance.
-
Any medications (especially anticoagulants or antiplatelets) will be reviewed and sometimes paused before surgery.
-
Optimize the child's condition: treat any lung infections, ensure good nutrition, ensure controlled heart function.
-
Families should ask the surgical/cardiology team about: what to expect in ICU, expected length of stay, feeding and activity progress, follow-up plan, signs of complications.
-
Psychosocial preparation: discussion with the team about long-term plan, additional surgeries, rehabilitation and support.
10. Is the BD Glenn procedure covered by insurance?
Yes. The BD Glenn surgery is generally covered by most major health insurance plans and government health programs, as it is a medically necessary, life-saving procedure for congenital heart disease.
Coverage usually includes:
-
Surgeon and hospital charges.
-
Intensive care costs.
-
Anesthesia and medications.
Parents should confirm coverage, preauthorization, and postoperative follow-up costs with their insurance provider and hospital before surgery.


