Introduction to Vagus Nerve Stimulation
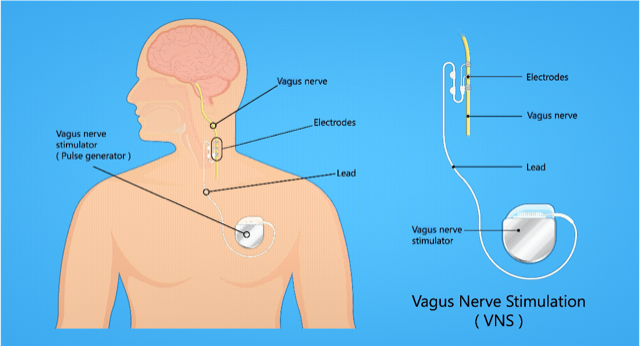
Vagus Nerve Stimulation (VNS) is a medical therapy that uses electrical impulses to stimulate the vagus nerve — one of the longest cranial nerves in the body (Cranial Nerve X) — to alter neural activity, with the goal of treating various neurological or psychiatric disorders. The approach falls under neuromodulation.
-
The vagus nerve extends from the brainstem through the neck, chest, and abdomen, innervating many organs (heart, lungs, digestive tract) and mediating autonomic functions (rest and digest).
-
The therapy is usually considered when standard treatments (medications, behavioral therapy, etc.) have been insufficient.
-
There are different kinds of VNS: implanted devices (surgically placed pulse generators and leads), and non-invasive/transcutaneous methods (e.g., hand-held or wearable stimulators) that stimulate via the skin or auricular branches.
Approved and Emerging Uses
-
Epilepsy (especially drug-resistant focal seizures) in both children (≥ age 4) and adults.
-
Treatment-resistant depression in adults who have not responded to multiple antidepressant treatments.
-
Stroke rehabilitation: VNS paired with physical/occupational therapy to restore function (especially in arm/hand) after ischemic stroke.
Causes and Risk of Vagus Nerve Stimulation
Here "causes and risk" is interpreted as why VNS is used (indications) and risks / who may not be a good candidate.
Indications (When VNS is Used)
-
Drug-Resistant Epilepsy
When two well-chosen, tolerated antiseizure medications have failed to control seizures adequately. -
Treatment-Resistant Major Depression
When multiple courses of antidepressants, psychotherapy, possibly electroconvulsive therapy have not worked. -
Post-Stroke Motor Impairment
For patients with moderate to severe loss of function in hand or arm, to enhance rehabilitation outcomes when paired with therapy. -
Other Investigational Uses (not yet fully approved)
Research is ongoing into VNS for conditions such as migraine, cluster headache, inflammatory conditions, PTSD, obesity, Alzheimer's etc.
Risks, Contraindications, and Who Should Be Cautious
-
Surgical Risks: As with any implanted device: risk of infection, pain at incision site, bleeding, risks due to anesthesia.
-
Stimulation-Related Side Effects: Hoarseness (voice changes), throat pain, cough, dysphagia (difficulty swallowing), shortness of breath. Often worse when stimulation is first turned on, then may improve over time.
-
Cardiac Concerns: Because the vagus nerve has effects on heart rate, individuals with conduction disorders or predisposition to bradycardia may have increased risk. Usually, VNS uses the left vagus nerve to reduce risk to cardiac function.
-
Sleep Apnea: May worsen existing sleep apnea. Need evaluation before implant.
-
Device-Related Risks: Lead malfunctions, battery failure, requirement for replacement surgeries.
Symptoms and Signs of Vagus Nerve Stimulation
Since VNS is a treatment, this section focuses on what patients might experience as symptoms or signs from the therapy, as well as what signs to monitor. Also what benefits and adverse effects show up.
Beneficial Effects (Signs of Success)
-
Reduced frequency, severity, or duration of epileptic seizures in epilepsy patients.
-
Improvement of mood, decreased depressive symptoms over months in treatment-resistant depression.
-
Improved motor function (better hand or arm movement) when VNS is used with rehabilitation after stroke.
-
Improved quality of life: better alertness, possibly improved sleep, fewer side-effects of medications, less depression in epilepsy.
Adverse Signs / Side Effects
-
Voice changes — hoarseness, altered voice quality, especially during stimulation pulse periods.
-
Throat-related discomfort — soreness, painful swallowing or cough.
-
Respiratory changes — mild shortness of breath or exacerbation of preexisting respiratory conditions.
-
Sleep disruption — sleep apnea or worsened breathing during sleep in some users.
-
Surgical complications signs: infection, swelling, pain at implantation site; hematoma.
-
In rare cases, permanent damage to vocal cords or nerve branches if electrode placement or stimulation is problematic.
Diagnosis of Suitability for Vagus Nerve Stimulation
Before VNS is used, there is a process to evaluate suitability, baseline assessments, and planning.
Evaluation Steps
-
Clinical History
-
Duration and severity of condition (e.g. frequency of seizures, severity of depression)
-
Prior treatments tried (drug therapies, psychotherapy, other neuromodulation)
-
Comorbid medical conditions (heart disease, lung disease, sleep apnea)
-
-
Physical Exam
-
ENT/voice exam (to check baseline vocal function)
-
Cardiovascular assessment (ECG to check conduction, heart rate response)
-
Neurological exam
-
-
Baseline Investigations & Imaging
-
Brain imaging (MRI, CT) for epilepsy to identify structural lesions.
-
Psychiatric evaluation for depression (scales, severity, risk).
-
Sleep studies if suspicion of sleep apnea.
-
-
Device Suitability & Counseling
-
Discussions with patient about expected benefits, trial periods, possible side effects.
-
Review of lifestyle, capacity to attend follow-ups, programming visits.
-
-
Device Testing / Programming
-
After implantation, periods of trial stimulation with adjustments.
-
Monitoring response over weeks to months to see reduction in symptoms.
-
Treatment Options of Vagus Nerve Stimulation
This section covers how VNS is delivered (device types), procedural details, parameter settings, and combinations with other treatments.
Types of VNS Devices
-
Implanted VNS: Pulse generator implanted under chest skin; lead attached to left vagus nerve in neck.
-
Non-invasive / Transcutaneous VNS (tVNS / auricular VNS): Stimulation via skin surface (neck or ear) without surgery. Useful for headache, migraine, and investigational use in other disorders.
Procedure & Programming
-
Surgical implantation: Typically outpatient; under general or regional anesthesia. Generator is placed in chest; lead tunneled to vagus nerve.
-
Initial settings: Low stimulation strength, frequency, duty cycles (“on” time vs “off” time). Gradually increased based on tolerance and response.
-
Patient controls: Some systems allow a magnet that the patient can use to trigger extra stimulation in certain contexts (e.g. aura before seizure).
Combination with Other Therapies
-
VNS is not usually a stand-alone cure (especially in epilepsy or depression) but used alongside medications, behavioral therapy, rehabilitation. In stroke rehab, pairing VNS with physical or occupational therapy amplifies recovery.
Emerging and Experimental Options
-
Enhanced stimulation protocols, closed-loop stimulation (sensing devices that respond to biomarkers) are being studied.
-
Expanded use in other disorders: gastrointestinal, inflammatory, psychiatric, neurodegenerative.
Prevention and Management of Vagus Nerve Stimulation
Because VNS itself is a therapy rather than a disease, “prevention” refers to preventing complications, ensuring optimal results, and managing side effects.
Best Practices to Minimize Risk
-
Proper patient selection
-
Pre-operative screening (heart, airway, existing respiratory issues, sleep apnea)
-
Use of experienced surgical teams
-
Correct placement of electrode leads
Managing Side Effects
-
Start stimulation at low settings; gradually increase to avoid acute adverse effects (voice changes, cough)
-
If side effects persist, reprogramming: adjust pulse width, frequency, off-time, or even temporarily turning off the device.
-
Regular follow-up to monitor battery status, lead integrity
Enhancing Therapeutic Outcomes
-
Ensure concomitant therapies (medication, rehab) are optimized
-
Patient education: what to expect, how to report symptoms or side effects
-
Periodic assessment of efficacy (e.g. seizure logs, depression rating scales, motor function scales)
Complications of Vagus Nerve Stimulation
While generally safe and well tolerated, VNS carries both immediate and long-term potential complications.
Surgical or Device-Related Complications
-
Infection at implant site
-
Hematoma or bleeding
-
Pain or discomfort at chest/neck incisions
-
Lead displacement or fracture requiring revision surgery
Stimulation-Related Adverse Effects
-
Hoarseness, voice alteration
-
Throat pain, cough, or voice fatigue
-
Difficulty swallowing (dysphagia)
-
Shortness of breath, especially in those with pulmonary issues
-
Sleep apnea worsening
-
Skin irritation around non-invasive device sites
Other Risks
-
Interaction with other implanted devices (e.g. pacemakers) — though VNS devices are designed to avoid cardiac interference when using left vagus nerve.
-
Battery failure, need for replacement every few years depending on usage
-
In some cases lack of efficacy — patients may not respond or may only have partial benefit
Living with the Condition of Vagus Nerve Stimulation
This section helps patients understand what life is like after getting VNS, what to expect, and how to monitor progress and challenges.
What to Expect After Implant
-
Recovery period: Mild pain/discomfort at surgical site, swelling. Usually resolves in days to weeks.
-
Device activation: Typically several weeks after surgery to allow healing. Gradual ramp-up of stimulation.
-
Follow-ups: Regular visits for programming, assessment of symptoms, side effects.
Monitoring Progress
-
Keep seizure diaries (if for epilepsy) noting frequency, severity, duration.
-
Depression scales or mood tracking for those treated for depression.
-
Physical therapy logs for stroke recovery.
Coping with Side Effects
-
Voice issues or throat discomfort may improve; voice therapy if needed.
-
Adjust lifestyle: avoid straining voice, avoid exposure to cold or irritants that trigger throat symptoms.
-
Sleep hygiene and possibly CPAP for sleep apnea
Quality of Life and Support
-
Many patients report improved mood, better ability to focus, less need for emergency treatments.
-
Psychological support is essential in mood disorders.
-
Support groups or peer networks can help with shared experiences and coping.
Long-Term Outlook
-
VNS does not cure epilepsy or depression, but can significantly reduce symptom burden.
-
Time to response can be months to over a year. Patience and persistence with programming often yield better results.
-
Device longevity: battery life varies (often 5-10+ years depending on settings), so revisions needed.
Top 10 Frequently Asked Questions about Vagus Nerve Stimulation (VNS)
1. What is Vagus Nerve Stimulation (VNS)?
Vagus Nerve Stimulation (VNS) is a medical treatment that uses mild electrical impulses to stimulate the vagus nerve, a key nerve that connects the brain to various organs in the body. A small device, similar to a pacemaker, is implanted under the skin in the chest and connected to the left vagus nerve in the neck. VNS is mainly used to treat epilepsy, treatment-resistant depression, and certain neurological or inflammatory conditions.
2. What conditions can VNS treat?
VNS has been approved and researched for several medical conditions:
-
Epilepsy - to reduce the frequency and severity of seizures.
-
Treatment-resistant depression - for patients who do not respond to medications or therapy.
-
Cluster headaches and migraines (non-invasive VNS devices are sometimes used).
-
Ongoing research explores its role in conditions such as Alzheimer's disease, anxiety disorders, rheumatoid arthritis, and Crohn's disease.
3. How does Vagus Nerve Stimulation work?
The vagus nerve plays a major role in regulating mood, inflammation, digestion, and brain activity. The implanted device delivers gentle, regular electrical pulses to the vagus nerve, which then sends signals to the brain. These signals can:
-
Reduce abnormal electrical activity in epilepsy.
-
Regulate mood-related brain regions in depression.
-
Influence inflammation and immune responses in ongoing studies.
4. Who is a candidate for VNS therapy?
VNS is usually recommended for:
-
Patients with drug-resistant epilepsy, who have not improved with at least two anti-seizure medications.
-
Individuals with treatment-resistant depression who have tried multiple therapies without relief.
-
Patients not suitable for brain surgery but still needing advanced therapy.
Doctors carefully evaluate medical history, current treatments, and risks before recommending VNS.
5. How is the VNS device implanted?
The procedure is performed under general anesthesia and usually takes 1-2 hours:
-
A small incision is made in the upper chest to implant the device.
-
A thin wire (lead) is tunneled under the skin and attached to the left vagus nerve in the neck.
-
The device is programmed externally after surgery to deliver electrical pulses at regular intervals.
Patients usually go home the same or next day, with full recovery in about 1-2 weeks.
6. What are the benefits of Vagus Nerve Stimulation?
-
Epilepsy: Reduces seizure frequency by 30-50% in many patients.
-
Depression: Improves mood in individuals unresponsive to medications.
-
Non-drug option: Provides relief when medicines cause side effects or fail.
-
Long-term benefits: Some patients see continued improvement over months or years.
7. What are the risks or side effects of VNS?
Most side effects are mild and often improve over time. They may include:
-
Hoarseness or voice changes
-
Throat discomfort or cough
-
Shortness of breath during stimulation
-
Tingling in the neck
Rare but possible complications include infection, device malfunction, or surgical risks.
8. Can VNS cure epilepsy or depression?
No. VNS is not a cure, but it can significantly reduce symptoms and improve quality of life. Many epilepsy patients experience fewer and less severe seizures, while some depression patients report improved mood and daily functioning. However, results vary, and ongoing follow-up is essential.
9. How long does a VNS device last, and can it be adjusted?
The battery of a VNS device typically lasts 5-10 years, depending on
usage settings. When the battery runs out, a minor surgery is needed to replace the
generator.
Doctors can adjust stimulation settings externally with a handheld
programmer, allowing personalization of therapy for each patient.
10. Is Vagus Nerve Stimulation available everywhere?
VNS is widely available in many countries but requires specialized centers with neurosurgeons and neurologists experienced in the procedure. Some non-invasive (external) VNS devices have also been approved in certain regions for migraines and cluster headaches. Patients should consult their doctor about availability, cost, and insurance coverage in their location.


